Sign up for daily news updates from CleanTechnica on email. Or follow us on Google News!
Hydrogen is often cited for its high energy density by mass — approximately 120 MJ/kg — making it appear to be an ideal energy carrier. However, this figure is frequently misunderstood or presented out of context, leading to misleading conclusions about hydrogen’s suitability for real-world energy storage and transportation. The issue lies not in its theoretical mass-based energy content but in the practical challenges associated with its volumetric energy density, storage requirements, and overall system efficiency.
This is a companion article to the Cranky Stepdad vs Hydrogen for Energy material. In a similar manner to John Cook’s Skeptical Science, the intent is a rapid and catchy debunk, a second level of detail in the Companion to Cranky Stepdad vs Hydrogen for Energy, and then a fuller article as the third level of detail.

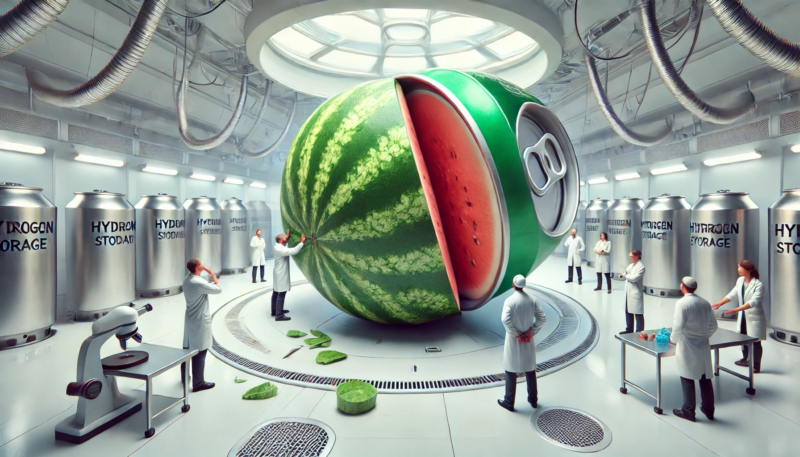
Hydrogen may weigh less, but storing it is like trying to squeeze a watermelon into a soda can.
Under standard atmospheric conditions, hydrogen is a very light gas with extremely low energy density by volume — about 0.01 MJ/L compared to gasoline’s 34.2 MJ/L. To be stored in usable quantities, hydrogen must be either compressed to extremely high pressures (typically 350 to 700 bar), liquefied at cryogenic temperatures (−253°C), or chemically bound in carriers such as ammonia or metal hydrides. Each of these options introduces significant energy penalties and complexity.
Compression to 700 bar — the pressure used in most hydrogen fuel cell vehicle tanks — requires about 10–15% of the energy content of the hydrogen itself just for the compression process (U.S. Department of Energy, 2023). Liquefaction is even more energy-intensive, consuming between 30–40% of hydrogen’s energy content (International Energy Agency [IEA], 2021). Chemical carriers introduce conversion and reconversion steps, further reducing overall efficiency.
These storage and transport challenges result in a substantially lower system-level energy efficiency for hydrogen than is often acknowledged. Bertuccioli et al. (2014) emphasize that while hydrogen can theoretically store energy efficiently by mass, real-world applications involve infrastructure that significantly erodes these gains. Lifecycle assessments show hydrogen’s round-trip efficiency — factoring in production via electrolysis, compression or liquefaction, transport, and final conversion back to electricity — can be as low as 25–35% (Qiu et al., 2021).
In contrast, lithium-ion batteries, while heavier, achieve round-trip efficiencies of 85–95% and do not require high-pressure or cryogenic infrastructure. Electric vehicle (EV) drivetrains using batteries are typically over twice as energy efficient as those relying on hydrogen fuel cells when measured from source to wheel (European Commission, 2022).
This issue is compounded when comparing infrastructure costs and complexity. Hydrogen requires pipelines designed to handle high diffusivity and embrittlement, or alternatively, expensive overland transport via cryogenic trucks or pressurized containers. These distribution challenges further reduce hydrogen’s competitiveness relative to direct electrification.
The widespread use of gravimetric energy density as a headline figure for hydrogen’s capabilities is therefore misleading — a textbook case of the misleading statistics fallacy. While technically correct, this single metric is divorced from the realities of how hydrogen must be stored, transported, and utilized. Bossel (2006) was among the first to comprehensively highlight this issue, arguing that hydrogen is “not an energy source, but a synthetic energy carrier with fundamental disadvantages.”
Hydrogen is essential as an industrial feedstock, for example in the manufacturing of ammonia fertilizers. However, for transportation, heat and energy storage applications, its low volumetric energy density and high infrastructure demands present major barriers to widespread deployment.
The energy content of a fuel by mass is only one piece of the puzzle. When volume, efficiency, infrastructure requirements, and lifecycle costs are included in the analysis, hydrogen isn’t viable as an energy carrier.
References:
Bertuccioli, L., Chan, A., Hart, D., Lehner, F., Madden, B., & Standen, E. (2014). The limitations of hydrogen as an energy storage medium. International Journal of Hydrogen Energy, 39(36), 21647–21662.
Bossel, U. (2006). Does a hydrogen economy make sense? Proceedings of the IEEE, 94(10), 1826–1837.
European Commission. (2022). Hydrogen Storage and Transport: Technical and Economic Barriers. Brussels: European Union.
International Energy Agency. (2021). The Future of Hydrogen: Storage and Transport Challenges. Paris: IEA.
Qiu, Y., Wang, L., Zhang, X., & Ding, Y. (2021). Comparing hydrogen storage with alternative energy carriers: A lifecycle efficiency assessment. Energy Reports, 7, 3950–3962.
U.S. Department of Energy. (2023). Hydrogen Storage and Distribution: Assessing Efficiency and Costs. Washington, DC: DOE.
Hume, N. (2021, October 4). Hydrogen’s energy density problem: Why storage and transport remain key obstacles. Financial Times.
Whether you have solar power or not, please complete our latest solar power survey.
Chip in a few dollars a month to help support independent cleantech coverage that helps to accelerate the cleantech revolution!
Have a tip for CleanTechnica? Want to advertise? Want to suggest a guest for our CleanTech Talk podcast? Contact us here.
Sign up for our daily newsletter for 15 new cleantech stories a day. Or sign up for our weekly one if daily is too frequent.
CleanTechnica uses affiliate links. See our policy here.
CleanTechnica’s Comment Policy