Sign up for daily news updates from CleanTechnica on email. Or follow us on Google News!
Last Updated on: 21st March 2025, 03:25 pm
Supramolecular chemistry, also known as “chemistry beyond the molecule,” focuses on the study of molecular recognition and high-order assemblies formed by noncovalent interactions. It’s in the news lately due to a study that focuses on solving the problem of plastic degradation into microplastics. Supramolecular plastics — polymers with structures held together by reversible interactions — are as strong as conventional plastics and biodegradable, but what makes them special is that they break down in seawater. The new plastic is, therefore, expected to help reduce harmful microplastic pollution that accumulates in oceans and soil and eventually enters the food chain.
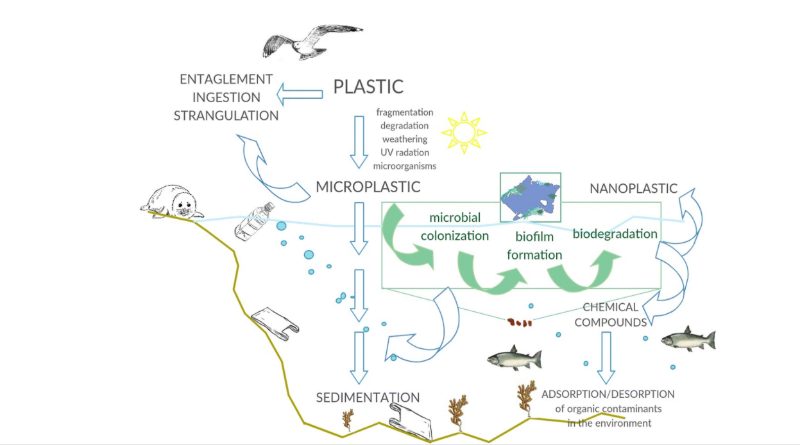
Every year, manufacturers across the globe produce plastic resins and fibers at a scale of hundreds of millions of metric tons. Larger plastics never completely disintegrate to their constituent parts; they simply become smaller bits of plastic. Products made from those materials degrade in the environment into increasingly smaller pieces that humans and animals can ingest or inhale. These microplastics not only course from rivers to ocean but also reside in every ecosystem.
Over 460 million metric tons of plastic are produced every year for use in a wide variety of applications. An estimated 20 million metric tons of plastic litter end up in the environment every year. That amount is expected to increase significantly by 2040. Plastic pollution affects all land, freshwater, and marine ecosystems. It is a major driver of biodiversity loss and ecosystem degradation and contributes to climate change.
Traditional plastics are non-sustainable and harm the environment. Scientists have been trying to develop safe and sustainable materials that can replace traditional plastics. While some recyclable and biodegradable plastics exist, the problem with current biodegradable plastics like PLA is that they often find their way into the ocean. Biodegradable plastics cannot be degraded because they are water insoluble. As a result, microplastics — plastic bits smaller than 5 mm — are harming aquatic life and finding their way into the food chain, including our own bodies.
The plastic crisis requires drastic measures, especially for the plastics’ end-of-life. The degradation of plastics through biological processes is of great significance for ecological health, so the feasibility of plastic degradation has attracted a lot of attention.
Plastics that can metabolize in oceans are highly sought for a sustainable future. Metabolism — the biochemical reactions that keep the cell alive and energized — is important in the study of plastics. A November 2024 study establishes the viability of plastics that are mechanically strong yet metabolizable under biologically relevant conditions owing to their dissociative nature with electrolytes. Led by Takuzo Aida at the RIKEN Center for Emergent Matter Science (CEMS), a research team has developed a durable plastic that won’t contribute to microplastic pollution in our oceans.
The RIKEN researchers applied salt-bridging sodium hexametaphosphate with di- or tritopic guanidinium sulfate in water, and the solution formed a cross-linked supramolecular network, which was stable unless electrolytes are resupplied. This unusual stability was caused by a liquid-liquid phase separation that expels sodium sulfate, generated upon salt bridging, into a water-rich phase. The two ionic monomers formed cross-linked salt bridges, which provide strength and flexibility.
“While the reversable nature of the bonds in supramolecular plastics have been thought to make them weak and unstable,” lead researcher Aida told Phys.org, “our new materials are just the opposite.”
Drying the remaining condensed liquid phase yielded glassy plastics that are thermally reshapable, such as thermoplastics, and usable even in aqueous media with hydrophobic parylene C coating. In the initial tests, one of the monomers was a common food additive called sodium hexametaphosphate and the other was any of several guanidinium ion-based monomers. Both monomers can be metabolized by bacteria, ensuring biodegradability once the plastic is dissolved into its components.
The “desalting” turned out to be the critical step; without it, the resulting dried material was a brittle crystal, unfit for use. Resalting the plastic by placing it in salt water caused the interactions to reverse and the plastic’s structure destabilized in a matter of hours. Thus, having created a strong and durable plastic that can still be dissolved under certain conditions, the researchers next tested the plastic’s quality.
The new plastics are non-toxic and non-flammable, meaning no CO2 emissions, and can be reshaped at temperatures above 120°C like other thermoplastics. By testing different types of guanidinium sulfates, the team was able to generate plastics that had varying hardnesses and tensile strengths, all comparable or better than conventional plastics. This means that the new type of plastic can be customized for need; hard scratch resistant plastics, rubber silicone-like plastics, strong weight-bearing plastics, or low tensile flexible plastics are all possible. The researchers also created ocean-degradable plastics using polysaccharides that form cross-linked salt bridges with guanidinium monomers. Plastics like these can be used in 3D printing as well as medical or health-related applications.
Lastly, the researchers investigated the new plastic’s recyclability and biodegradability. After dissolving the initial new plastic in salt water, they were able to recover 91% of the hexametaphosphate and 82% of the guanidinium as powders, indicating that recycling is easy and efficient. In soil, sheets of the new plastic degraded completely over the course of 10 days, supplying the soil with phosphorous and nitrogen similar to a fertilizer.
“With this new material, we have created a new family of plastics that are strong, stable, recyclable, can serve multiple functions, and importantly, do not generate microplastics,” says Aida.
It was determined that this approach can be extended to polysaccharide-based supramolecular plastics that are applicable for three-dimensional printing. The experimental findings were published in Science.
Final Thoughts
In another positive sign for those concerned about human health and ocean pollution, the SEC sided with As You Sow over Pepsi Co on a shareholder resolution urging the soda and food giant to reduce plastic pollution. The SEC’s decision is the second plastic pollution-related proposal from As You Sow to overcome a no-action challenge despite stricter guidance from the new administration.
Pepsi’s stated commitments to sustainable packaging ring hollow when more than 18% of its packaging remains in non-recyclable flexible packaging, says As You Sow. Flexible packaging is one of the fastest growing packaging sectors and a major contributor to global plastic pollution.
Whether you have solar power or not, please complete our latest solar power survey.
Chip in a few dollars a month to help support independent cleantech coverage that helps to accelerate the cleantech revolution!
Have a tip for CleanTechnica? Want to advertise? Want to suggest a guest for our CleanTech Talk podcast? Contact us here.
Sign up for our daily newsletter for 15 new cleantech stories a day. Or sign up for our weekly one if daily is too frequent.
CleanTechnica uses affiliate links. See our policy here.
CleanTechnica’s Comment Policy